| Availability: | |
|---|---|
| Quantity: | |
JONENG








316L Sanitary Grade Multi-channel Biopharmaceutical Manual Diaphragm Valve
The 316L Sanitary Grade Multi-channel Biopharmaceutical Manual Diaphragm Valveis a hygienic valve designed for multi-path fluid distribution and precise manual control. Made from 316L stainless steel, it ensures sterility, corrosion resistance, and reliable sealing, making it ideal for biopharmaceutical, biotech, and high-purity processing systems requiring contamination-free flow handling.
Working Principles

The 316L Sanitary Grade Multi-channel Biopharmaceutical Manual Diaphragm Valve operates by manually adjusting a handwheel or handle to press the diaphragm onto the valve seat, thereby controlling fluid flow. When the actuator is turned, it moves a stem that flexes the diaphragm upward or downward, allowing one or multiple channels to open, close, or redirect flow. The diaphragm forms the only barrier between the fluid and the actuator, ensuring sterility and preventing contamination. Its multi-channel design enables complex flow paths such as sampling, distribution, mixing, or diversion within a compact valve body. This principle provides smooth flow transitions, reliable shutoff, and high cleanliness required in biopharmaceutical production.
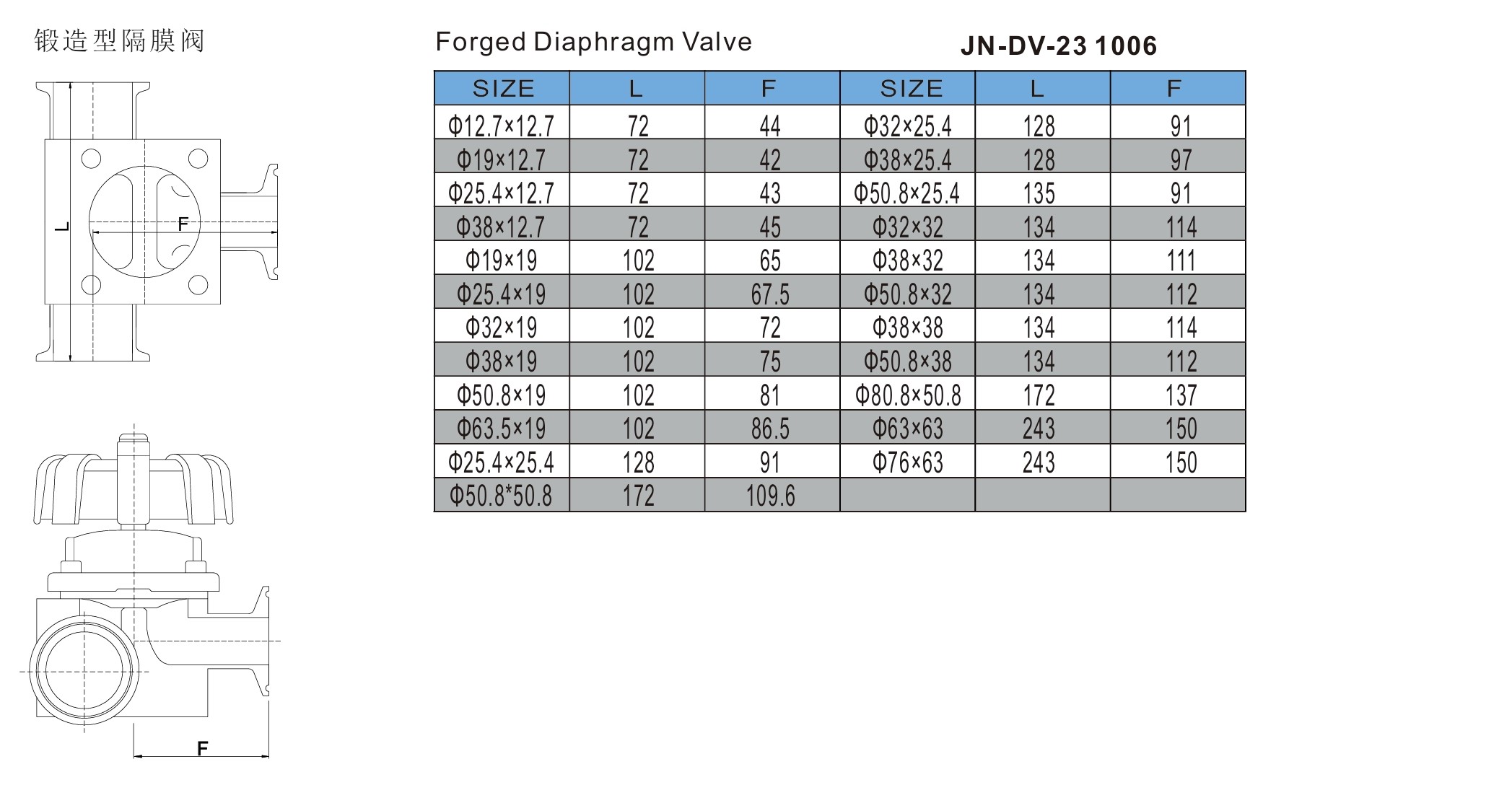
Stainless Steel Diaphragm Valve Specification Chart

| Parameter | Specification |
|---|---|
| Material | 316L Stainless Steel (≤0.6% Roughness Ra) |
| Valve Type | Multi-channel Manual Diaphragm Valve |
| Diaphragm Material | EPDM / PTFE / Silicone / TFM (FDA & USP Class VI compliant) |
| Operation Method | Manual Handwheel / Lever |
| Connection Type | Tri-Clamp / Welded / Aseptic Ferrule |
| Flow Paths | 2-way, 3-way, 4-way, or Custom Multi-port |
| Surface Finish | Electro-polished, Ra 0.4–0.6 μm (Sanitary Grade) |
| Temperature Range | -10°C to 150°C (depending on diaphragm) |
| Pressure Rating | Up to 10 bar (varies with size and diaphragm type) |
| Standards | ASME BPE, FDA, USP Class VI, GMP |
| Applications | Biopharmaceutical, Biotech, Aseptic Processing, High-purity Systems |
Features

Hygienic 316L Construction – Made from corrosion-resistant 316L stainless steel with ultra-smooth surfaces, ensuring sterility, easy cleaning, and compliance with strict biopharmaceutical hygiene standards.
Multi-channel Flow Design – Allows multiple flow paths in one compact body, enabling distribution, sampling, mixing, or diversion while reducing system complexity and installation space.
Manual Precision Control – The handwheel provides stable, precise actuation, allowing operators to manually regulate flow with high accuracy in sensitive bioprocess environments.
Aseptic Diaphragm Seal – The diaphragm isolates fluid from mechanical components, preventing contamination and ensuring reliable, sterile shutoff in high-purity systems.
Flexible Connection Options – Supports tri-clamp, weld, or aseptic ferrule connections, ensuring easy integration into biopharmaceutical piping systems with superior sealing and validation performance.
Application

The 316L Sanitary Grade Multi-channel Biopharmaceutical Manual Diaphragm Valve is widely used in industries that require strict sterility and precise flow control. It is commonly applied in biopharmaceutical production, such as vaccine manufacturing, cell culture systems, buffer preparation, and WFI distribution. It is also used in biotechnology, laboratory processes, and high-purity water systems, where contamination-free flow handling is essential
Working Principles

The 316L Sanitary Grade Multi-channel Biopharmaceutical Manual Diaphragm Valve operates by manually adjusting a handwheel or handle to press the diaphragm onto the valve seat, thereby controlling fluid flow. When the actuator is turned, it moves a stem that flexes the diaphragm upward or downward, allowing one or multiple channels to open, close, or redirect flow. The diaphragm forms the only barrier between the fluid and the actuator, ensuring sterility and preventing contamination. Its multi-channel design enables complex flow paths such as sampling, distribution, mixing, or diversion within a compact valve body. This principle provides smooth flow transitions, reliable shutoff, and high cleanliness required in biopharmaceutical production.
Feature and Specification


Stainless Steel Diaphragm Valve Specification Chart

| Material | 316L Stainless Steel (≤0.6% Roughness Ra) |
| Valve Type | Multi-channel Manual Diaphragm Valve |
| Diaphragm Material | EPDM / PTFE / Silicone / TFM (FDA & USP Class VI compliant) |
| Operation Method | Manual Handwheel / Lever |
| Connection Type | Tri-Clamp / Welded / Aseptic Ferrule |
| Flow Paths | 2-way, 3-way, 4-way, or Custom Multi-port |
| Surface Finish | Electro-polished, Ra 0.4–0.6 μm (Sanitary Grade) |
| Temperature Range | -10°C to 150°C (depending on diaphragm) |
| Pressure Rating | Up to 10 bar (varies with size and diaphragm type) |
| Standards | ASME BPE, FDA, USP Class VI, GMP |
| Applications | Biopharmaceutical, Biotech, Aseptic Processing, High-purity Systems |
Features

Hygienic 316L Construction – Made from corrosion-resistant 316L stainless steel with ultra-smooth surfaces, ensuring sterility, easy cleaning, and compliance with strict biopharmaceutical hygiene standards.
Multi-channel Flow Design – Allows multiple flow paths in one compact body, enabling distribution, sampling, mixing, or diversion while reducing system complexity and installation space.
Manual Precision Control – The handwheel provides stable, precise actuation, allowing operators to manually regulate flow with high accuracy in sensitive bioprocess environments.
Aseptic Diaphragm Seal – The diaphragm isolates fluid from mechanical components, preventing contamination and ensuring reliable, sterile shutoff in high-purity systems.
Flexible Connection Options – Supports tri-clamp, weld, or aseptic ferrule connections, ensuring easy integration into biopharmaceutical piping systems with superior sealing and validation performance.
Application

The 316L Sanitary Grade Multi-channel Biopharmaceutical Manual Diaphragm Valve is widely used in industries that require strict sterility and precise flow control. It is commonly applied in biopharmaceutical production, such as vaccine manufacturing, cell culture systems, buffer preparation, and WFI distribution. It is also used in biotechnology, laboratory processes, and high-purity water systems, where contamination-free flow handling is essential